a) Petroleum is highly flammable (fuel)
b) Sources of ignition may be present at various locations. (Ignition)
c) Oxygen provides the medium necessary to sustain any fire
The foregoing three form the essential sides of the fire triangle. When the three sides of a fire triangle join come together, we have fire. On a tanker we cannot isolate the petroleum as that is the cargo we carry, but we can prevent the oxygen and source of ignition from being present at the same time to complete the fire triangle.
When petroleum is ignited, it is the gas progressively given off by the liquid, which burns as a visible flame. The quantity of gas given off by petroleum liquid depends on its volatility, which is frequently expressed for purposes of comparison in terms of Reid vapour pressure.
Petroleum gases can be ignited and will burn only when mixed with air in certain proportions. If there is too little or too much petroleum gas the mixture cannot burn. For the gas mixtures from the petroleum liquids to ignite the overall range has to be from a minimum of about 1% gas by volume (known as Lower Flammable Limit – LFL) to a maximum of about 10% gas by volume in air (known as Upper Flammable Limit – UFL).
As petroleum liquid is heated the concentration of gas in air above it increases. The temperature of the liquid at which this concentration, using a specific measuring technique, reaches the LFL is known as the flashpoint of the liquid.
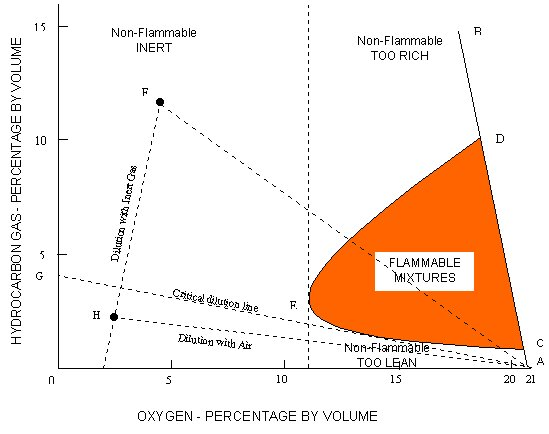
Hydrocarbon gas normally encountered in petroleum tankers cannot burn in an atmosphere containing less than approximately 11% oxygen by volume. Accordingly one way to provide protection against fire or explosion in the vapour space of cargo tanks is to keep the oxygen level below that figure.
The flammable limits vary for different pure hydrocarbon gases and for mixtures derived from different liquids. For practical purposes the lower and upper flammable limits of crude oil vapours are taken to be 1% and 10% respectively by volume. Points C and D on the line AB in the figure indicate these values.
Any point in the diagram represents mixtures of hydrocarbon gas, air and inert gas, specified in terms of hydrocarbon gas and oxygen contents. As inert gas is added to the hydrocarbon gas/air mixture, the flammable range decreases until a point, represented by E, is reached where LFL and UFL coincide. This point corresponds to oxygen content of approximately 11%. For practical purposes and to allow a safety margin, 8% is taken as the level of oxygen at which no hydrocarbon gas/air mixture can burn under any circumstances. To prevent fire or explosion in a tank containing hydrocarbon gas/air mixture it is therefore necessary to produce and supply inert gas having oxygen content not normally exceeding 5% and to displace the existing air in the tank until the resultant oxygen level throughout the tank does not exceed 8% by volume.
The diagram given above (Flammability Composition Diagram) can be considered the most important diagram to understand the concept of flammability.
Hydrocarbon gas normally encountered in petroleum tankers cannot burn in an atmosphere containing less than approximately 11% oxygen by volume. Accordingly one way to provide protection against fire or explosion in the vapour space of cargo tanks is to keep the oxygen level below that figure.
The flammable limits vary for different pure hydrocarbon gases and for mixtures derived from different liquids. For practical purposes the lower and upper flammable limits of crude oil vapours are taken to be 1% and 10% respectively by volume. Points C and D on the line AB in the figure indicate these values.
Any point in the diagram represents mixtures of hydrocarbon gas, air and inert gas, specified in terms of hydrocarbon gas and oxygen contents. As inert gas is added to the hydrocarbon gas/air mixture, the flammable range decreases until a point, represented by E, is reached where LFL and UFL coincide. This point corresponds to oxygen content of approximately 11%. For practical purposes and to allow a safety margin, 8% is taken as the level of oxygen at which no hydrocarbon gas/air mixture can burn under any circumstances. To prevent fire or explosion in a tank containing hydrocarbon gas/air mixture it is therefore necessary to produce and supply inert gas having oxygen content not normally exceeding 5% and to displace the existing air in the tank until the resultant oxygen level throughout the tank does not exceed 8% by volume.